ORIGINAL RESEARCH
Gut Microbiota Dysbiosis and Its Role in the Pathogenesis of Metabolic Dysfunction-Associated Steatotic Liver Disease
ABSTRACT
Background: Metabolic dysfunction-associated steatotic liver disease (MASLD) has become the most prevalent chronic liver disease in the world; an estimated 30–35% of the world's population is affected, and the prevalence of MASLD has been increasing in recent years. An increasing number of studies have indicated a close relationship between dysbiosis and MASLD. Hence, there is an interest in exploring fatty infiltration as a result of dysbiosis, which includes bacterial composition disturbance, with a profound analysis of preventive and therapeutic factors that impact the occurrence and progression of liver disease. This study aimed to analyze the gut microbiota composition in patients with MASLD, with the potential identification of diagnostic markers and protective factors.
Objectives: This study aimed to evaluate the gut microbiota composition in patients with MASLD and MASH, identify potential diagnostic and protective microbial markers, and investigate the role of dysbiosis in the development and progression of the disease.
Methods: This study involved 123 subjects divided into two groups (65 MASLD, 18 MASH, and 40 healthy individuals in the control group) with a median age of 49 (23–75) diagnosed with MASH/MASLD based on ultrasound and biochemical tests. The criteria for fatty infiltration included a diffuse increase in the echogenicity of the liver parenchyma, decreased attenuation of the liver, and a ratio between the brightness level of the liver and the right kidney, calculated for the hepato-renal index (HRI) determination. The biochemical evaluation included liver function tests and a lipid profile. Dysbiosis was assessed using a stool bacteriological test and a stool test for dysbiosis.
Results: The genus Enterococcus, like Streptococcus, was increased in patients with MASLD/MASH compared with controls. Additionally, uncultured Clostridiales, as well as entero-hemolytic Escherichia coli, were also increased. In contrast, genus Bifidobacterium and Lactobacillaceae were decreased in patients with MASLD/MASH. A significant loss of beneficial bacteria, such as Faecalibacterium, was observed, affecting intestinal barrier function. The diversity of the microbiota was decreased in patients compared with controls.
Conclusions: Our study revealed an association between the abundance of Bacteroides in the gut and MASLD/MASH. MASLD developed initially in patients with significant dysbiosis. These results demonstrate that gut microbiota analysis provides prognostic information in addition to classical risk factors for MASLD severity, and strongly suggest that the gut microbiota plays a significant role in the pathogenesis of MASLD. Patients with MASLD should be tested for dysbiosis, and vice versa. It also suggests that correcting the gut microbiome may be beneficial for treating patients with MASLD/MASH.
Keywords: Dysbiosis; gut microflora; metabolic dysfunction-associated steatotic liver disease (MASLD); metabolic dysfunction-associated steatohepatitis (MASH).
DOI: 10.52340/GBMN.2025.01.01.119
BACKGROUND
Metabolic dysfunction-associated steatotic liver disease (MASLD) is portrayed by deposition of fats in the hepatocytes that ranges from simple hepatosteatosis to steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma.1 Some studies have shown that alterations in the gut microbiota are related to obesity, insulin resistance (IR), type 2 diabetes, and MASLD.2-4 When there are no other reasons for secondary hepatic fat buildup, hepatic steatosis is an indicator of Metabolic dysfunction-associated steatotic liver disease.
Recent findings have shown that dysbiosis of gut microbiota also disturbs hepatic carbohydrate and lipid metabolism, affecting the balance between pro- and anti-inflammatory effectors in the liver, thus prompting MASLD and its progression to metabolic dysfunction-associated steatohepatitis (MASH).2,4,5 However, the comprehensive mechanism of this severe liver disease is so far mysterious. Numerous new features remain to be revealed, particularly in the specific functions of the gut microbiota. Resolving these issues would be a milestone in the treatment of MASLD and other gut microbiota-related syndromes.2 Therefore, it is necessary to completely recognize the pathogenesis of this disease and the role of gut microbiota in its progression, which may pave the way to discover new therapeutic strategies for control of MASLD.
There is a growing interest in healthy gut microbiota and the restoration of dysbiotic gut microbiota. The probiotics are commonly used as an appealing approach due to their extended traditional use, health-promoting properties, and enormous literature supports.6,7 Evidence suggests that probiotics can regulate intestinal microbiota and may be a preferred approach for preventing or treating MASLD and other chronic liver diseases.6 Several recent findings have shown that probiotics tend to change the dysbiosis gut microbiota to the normal and could be a potential treatment approach for the management of MASLD.6,7 Given the close anatomical and functional connection between the gut and liver, as well as microbial dysbiosis, MASLD, and the use of probiotics, these factors may modulate this dysbiosis.4,7
Global burden of MASLD
This trend is reflective of the worldwide increase in obesity and metabolic disorders.2,3 MASLD has been referred to as a silent epidemic, as it is mostly asymptomatic in the early stages and frequently underdiagnosed, with consequently delayed intervention.8 In the Western world, MASLD has emerged as a common cause of chronic liver disease and is becoming an important indication for liver transplantation.8 Moreover, its association with CVD and extrahepatic malignancies increases its clinical relevance, making it a significant public health priority.2,8
Pathophysiology of MASLD
The pathogenesis of MASLD is a complex and multifactorial process. The "multiple-hit hypothesis" has replaced the previous "two-hit hypothesis" as an explanation for the development of the disease.
Key factors contributing to the pathophysiology of MASLD include:
-
Insulin resistance: A major contributor to MASLD is insulin resistance, which leads to excessive fat storage in the liver. This disrupts normal metabolic processes, leading to increased production of free fatty acids and glucose, which further exacerbates liver dysfunction. Without intervention, insulin resistance can accelerate the progression of MASLD to more severe stages.1
-
Oxidative stress and inflammation: ROS induce oxidative stress, leading to hepatocyte injury and the subsequent stimulation of an inflammatory cascade. Resident liver macrophages also release pro-inflammatory cytokines, including TNF-α, thus further contributing to liver injury and the progression towards MASLD. Both oxidative stress and inflammation are interactive processes that are essential for the evolution of MASH from simple steatosis.1
-
Gut-liver axis: MASLD pathogenesis involves the gut-liver axis. The disturbance in gut microbiota composition and increased intestinal permeability enable the translocation of endotoxins, such as LPS, to the liver. Translocation induces further inflammation through the activation of TLRs, which are crucial for the innate immune response.2,9 Changes in the gut microbiome may impact the liver and indicate dietary modifications that are useful in the management of MASLD.4,5,10
-
Genetic and epigenetic factors: Besides polymorphisms in the PNPLA3 and TM6SF2 genes, genetic predisposition has been recognized as a decisive factor in susceptibility to MASLD and its aggravation. Epigenetic modifications can also influence gene expression and contribute to disease heterogeneity.1 The identification of genetic and epigenetic factors may provide bases for identifying individuals at risk and for developing selective therapies.
METHODS
The study was conducted at the Tbilisi State Medical University clinics in Tbilisi, Georgia. The study protocol was approved by the Ethics Committee of Tbilisi State Medical University, and written consent was obtained from all participants. Patients referred to our clinics for persistently elevated liver enzymes and clinical suspicion of MASLD were initially assessed as per standard of care to rule out other causes of liver disease. After persistently elevated alanine aminotransferase (ALT) levels, the diagnosis of MASLD was confirmed with abdominal ultrasound. During the initial visit, patients were invited to participate in this study. After providing written informed consent, they were instructed on how to collect and transport the stool sample.
The inclusion criteria were age >18 years and a diagnosis of MASLD or a healthy liver. Exclusion criteria included having any of the following conditions: Hepatitis, autoimmune disorders, advanced liver disease, cancers, irritable bowel syndrome, inflammatory bowel disease, chronic diarrhea, liver enzymes 10 times above the normal values, any gastrointestinal surgeries, alcohol use >40 g per week, use of corticosteroids, probiotics, Vitamin E and fish oil supplements within 6 months, use of antibiotics within 6 weeks and dieting within 1 month.
Fecal samples collection
A total of 123 stool samples from the study population were collected (83 from MASLD patients and 40 from the control group), and they were immediately transferred to the microbiology laboratory of the Bacteriophage Analytical Center. The samples were homogenized by agitation with a vortex and aliquoted within two hours of defecation. The aliquots were then instantly frozen and stored at − 80 °C in screw capped cryovial tubes until used for DNA extraction.
RESULTS
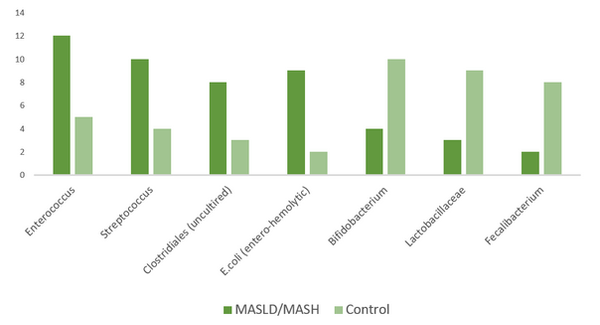
The genus Enterococcus, like Streptococcus, was increased in patients with MASLD/MASH compared with controls. Additionally, uncultured Clostridiales, as well as entero-hemolytic Escherichia coli, were also increased. In contrast, genus Bifidobacterium and Lactobacillaceae were decreased in patients with MASLD/MASH (Tab.1). A significant loss of beneficial bacteria, such as Faecalibacterium, was observed, which affected intestinal barrier function. The diversity of the microbiota was decreased in patients compared with controls. Alpha diversity was significantly lower in the MASLD/MASH group (p < 0.05), indicating reduced microbial richness and evenness (Fig.1).
TABLE 1. Microbiota changes in MASLD/MASH patients compared to controls

Abbreviations: MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease
FIGURE 1. Relative Abundance of Key Microbiota in MASLD/MASH vs. controls
DISCUSSION
This study demonstrates a clear association between gut microbiota dysbiosis and the presence of MASLD and MASH, highlighting significant compositional shifts that may contribute to the pathogenesis of these diseases.2,4 Notably, we observed a relative increase in pro-inflammatory or opportunistic bacterial taxa, such as Enterococcus, Streptococcus, uncultured Clostridiales, and entero-hemolytic Escherichia coli, in MASLD/MASH patients, accompanied by a marked reduction in protective genera, including Bifidobacterium, Lactobacillaceae, and Faecalibacterium (Tab.2). These alterations were accompanied by significantly reduced microbial diversity (α-diversity), a finding consistent with previous studies that have linked lower gut microbial richness to increased metabolic and inflammatory disease burden.2,11
TABLE 2. Clinical and biochemical characteristics of study groups

Abbreviations: MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease
The observed dysbiosis aligns with the "multiple-hit" hypothesis in MASLD, where metabolic dysfunction, oxidative stress, and gut-derived inflammation act synergistically to promote hepatic steatosis and progression to MASH.1 The reduction of beneficial commensals, such as Faecalibacterium, a prominent butyrate-producing genus with anti-inflammatory properties, may impair intestinal barrier integrity and promote systemic endotoxemia, thereby facilitating hepatic inflammation through toll-like receptor (TLR) activation.9,12 These changes further compromise hepatic lipid metabolism and immune balance, thereby accelerating MASLD progression.
The increased abundance of Enterococcus and Streptococcus genera commonly associated with small intestinal bacterial overgrowth (SIBO)—supports earlier findings that link SIBO with MASLD and its more severe forms.4 Likewise, elevated levels of entero-hemolytic E. coli may reflect increased mucosal permeability and immune activation, which are known to exacerbate hepatic injury.2,9 This gut-liver axis interplay reinforces the notion that intestinal dysbiosis is not merely a consequence of MASLD but a potential driver of its onset and advancement.
Our findings further suggest the diagnostic and prognostic utility of gut microbiota profiling in MASLD. The altered microbial composition and reduced diversity observed in MASLD patients offer potential for the development of non-invasive biomarkers to support risk stratification and early intervention.2,11 These insights are particularly valuable given the asymptomatic nature of early MASLD and the limitations of current diagnostic approaches.
Importantly, this study supports the growing interest in microbiome-targeted therapies, such as probiotics and prebiotics, for the management of MASLD. Restoring microbial homeostasis may enhance intestinal barrier function, reduce hepatic inflammation, and mitigate disease progression. Our results provide a compelling rationale for integrating microbiota assessment into routine MASLD evaluation and for exploring therapeutic strategies that aim to modulate the gut-liver axis.
In conclusion, the present study underscores the significance of gut microbiota alterations in MASLD and MASH, highlighting the diagnostic potential of dysbiosis and the promise of microbiota-directed interventions. Future longitudinal studies are warranted to establish causal relationships and assess the efficacy of targeted microbial therapies.
CONCLUSIONS
Our study revealed an association between the abundance of Bacteroides in the gut and MASLD/MASH. MASLD initially developed in patients with significant dysbiosis. These results demonstrate that gut microbiota analysis provides prognostic information in addition to classical risk factors for MASLD severity, and strongly suggest that the gut microbiota plays a significant role in the pathogenesis of MASLD. Patients with MASLD should be tested for dysbiosis, and vice versa. These findings also suggest that correcting the gut microbiome may be beneficial for treating patients with MASLD/MASH. These results indicate that gut microbiota profiling offers prognostic insights beyond traditional metabolic risk factors and may enhance risk stratification in MASLD. Routine assessment of dysbiosis in MASLD patients, along with consideration of hepatic involvement in individuals with disrupted microbiota, could improve clinical management. Moreover, the data support the therapeutic potential of microbiome modulation as an adjunctive approach in the treatment of MASLD/MASH
AUTHOR AFFILIATION
1 Department of Internal Medicine, Tbilisi State Medical University, Tbilisi, Georgia
2 Rayman Clinic, Tbilisi, Georgia
3 Enmedic Clinic, Tbilisi, Georgia
REFERENCES
-
Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012.
-
Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17(5):279–297. doi: 10.1038/s41575-020-0269-9.
-
Bashiardes S, Shapiro H, Rozin S, Shibolet O, Elinav E. Non-alcoholic fatty liver and the gut microbiota. Mol Metab. 2016;5(9):782–794. doi: 10.1016/j.molmet.2016.06.003.
-
Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412–425. doi: 10.1038/nrgastro.2016.85.
-
Kolodziejczyk AA, Zheng D, Elinav E. Diet–microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17(12):742–753. doi: 10.1038/s41579-019-0256-8.
-
Derakhshani H, Naserian S, Vakili S, Momtazi-Borojeni AA, Bosi A, Hajizadeh MR, et al. Probiotic supplementation in patients with NAFLD: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2020;35(5):899–907. doi: 10.1111/jgh.14961.
-
Loman BR, Hernández-Saavedra D, An R, Rector RS. Prebiotic and probiotic treatment of MASLD: A systematic review and meta-analysis. Nutrients. 2018;10(10):1339. doi:10.3390/nu10101339.
-
Eslam M, Sanyal AJ, George J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312.
-
Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, et al. Hepatic injury in nonalcoholic steatohepatitis contributes to altered intestinal permeability. Cell Mol Gastroenterol Hepatol. 2015;1(2):222–232. doi: 10.1016/j.jcmgh.2015.01.006.
-
Milosevic I, Vujovic A, Barac A, Djukic A, Aksamija G, Korac M, et al. Gut-liver axis, gut microbiota, and its modulation in the management of liver diseases: A review of the literature. Int J Mol Sci. 2019;20(2):395. doi: 10.3390/ijms20020395.
-
Del Chierico F, Nobili V, Vernocchi P, Russo A, Stefanis CD, Gnani D, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65(2):451–464. doi: 10.1002/hep.28572.
