CLINICAL CASE
Bone Marrow-Derived Stem Cell Transplantation in Optic Nerve Atrophy
Nana Digmelashvili1,2, Merab Dvali3,4, Natia Gamkrelidze5, Natalia Pavliashvili5, Natalia Maghlakelidze6,7, Nino Kraveishvili8,
Konstantin Mardaleishvili2, George Loladze2, Giorgi Kharebava2,6, Nikoloz Jincharadze9, Irakli Zakroshvili10, Dimitri Mirvelashvili11,
Maria Fadeecheva12, Ana Dzolashvili1
ABSTRACT
This study aimed to evaluate the safety and feasibility of human bone marrow-derived stem cell transplantation in a single patient with optic nerve atrophy. A total of 735 million bone marrow-derived stem cells were isolated. Of these, 490 million cells were administered intrathecally, 15 million cells were injected into each vitreous cavity, 15 million cells were injected into each episcleral region, and 72.5 million cells were injected into each retrobulbar space. As a result, visual acuity improved from 0.1 to 0.4 at six months post-transplantation and subsequently decreased; however, vision remained superior to baseline values. Comparable improvements were noted in visual field assessments and electroretinogram (ERG) findings. In conclusion, autologous bone marrow-derived stem cell transplantation in patients with optic nerve damage demonstrated transient improvements in visual function.
Keywords: Bone marrow-derived stem cells; optic nerve atrophy; stem cells.
DOI: 10.52340/GBMN.2025.01.01.126
INTRODUCTION
Optic nerve atrophy (ONA) is the endpoint of numerous optic neuropathies, characterized by the degeneration of retinal ganglion cells (RGCs) and their axons, leading to partial or complete loss of vision.1 The optic neuropathy may be: ischemic, traumatic, glaucomatous, inflammatory, toxic, compression, hereditary, or tumor-induced. Histopathologically, it manifests as axonal loss, gliosis, and pallor of the optic disc.1 Conventional treatments are focused on preventing progression rather than reversing neuronal damage, underscoring the urgent need for regenerative strategies aimed at restoring lost function.2,3
Recent advancements in stem cell therapy include the development of RGC organoids, CRISPR-mediated gene editing in iPSCs for inherited conditions such as Leber hereditary optic neuropathy (LHON) and dominant optic atrophy (DOA), and intravitreal or subretinal injections of MSCs for their paracrine support.4-6 Beike Biotechnology reports positive anecdotal outcomes from the clinical use of umbilical cord-derived stem cells, indicating improvements in visual acuity and field in certain patients. However, thorough clinical trials are still needed to substantiate these claims.7 While no stem cell-based therapy has yet received widespread regulatory approval for ONA, ongoing trials and translational research continue to shed light on the safety and potential efficacy of these approaches.3,8 iPSCs, due to their patient-specific origin and versatility in differentiation, represent a cornerstone of personalized regenerative medicine for optic neuropathies.3,8 Future research should focus on refining transplantation methods to ensure long-term cell survival and integration, as well as minimizing immunogenicity and tumorigenic risks.8,9 These goals will likely be achieved through interdisciplinary collaborations that integrate stem cell biology, gene editing, biomaterials, and neuro-ophthalmology.
Emerging therapies involving stem cells—particularly induced pluripotent stem cells (iPSCs) and mesenchymal stem cells (MSCs) - hold promise for neuroregeneration and neuroprotection in ONA. These cells demonstrate potential in differentiating into RGC-like phenotypes, integrating into host tissue, and secreting trophic factors essential for neuronal survival.2,3 Preclinical studies have shown that iPSCs can recapitulate disease phenotypes in vitro, providing a platform for modeling optic neuropathies and evaluating gene and cell therapies.2,3 Furthermore, MSCs possess immunomodulatory and neuroprotective capabilities, making them attractive candidates for halting optic nerve degeneration.4
Stem cell therapy can replace damaged cells directly by implanting exogenous cells into the affected area, which are also capable of promoting the proliferation, differentiation, and restoration of tissue-resident cells. This is done by modifying their microenvironments via the activation of precursor cells.10,1 The nerve grafting is gold standard treatment for peripheral nerve injury for now, but this has issues such as donor site morbidity. New techniques focus on replacing these grafts with growth factors and/or stem cells (MSCs). Dental-MSCs may also be the potential sources of MSCs for nerve repair.11,12
Bone marrow-derived stromal cells (BM-MSCs) are gaining increasing importance in the field of regenerative medicine. Although the therapeutic value of MSCs is now being established through numerous clinical trials, concerns have been raised regarding their expansion under regulatory guidelines. Bone marrow-derived MSCs (BM-MSCs) account for approximately 0.001-0.01% of bone marrow mononuclear cells. Due to their low abundance, extensive in vitro culturing and expansion are necessary to obtain sufficient numbers for research or clinical applications. The properties of BM-MSCs - such as ease of isolation from bone marrow without causing an immunological problem, ability to expand in vitro within a short period, biopreservation for point-of-care delivery with minimal loss of potency, and the absence of reported severe adverse reactions during autologous or allogenic therapy - make them an essential tool in regenerative medicine.12
While significant progress has been made in the field of optic nerve regeneration over the past few decades, it is essential to recognize that the process of axon regeneration is highly complex, and relying on a single factor may not be sufficient to facilitate complete regeneration. Providing a combinatorial treatment is almost certainly necessary to achieve successful regeneration of the optic nerve, and numerous studies investigating different combinations have demonstrated that combining treatments results in more regeneration compared to single therapies. While these treatments have shown promising results in experimental models, treatments focused solely on optic nerve regeneration are still in the early stages of preclinical development. Ultimately, the ability to regenerate the axons of the optic nerve would enable the development of successful therapies for the millions of people who suffer from optic neuropathies.13
CASE
A 25-year-old male presented with bilateral optic nerve atrophy and reduced visual acuity (VOD:0.1; VOS:0), along with ataxia and frequent headaches. The patient had a history of meningitis at the age of 4 years, which resulted in persistent visual impairment and cerebellar ataxia.
In 2022, a brain MRI revealed hypoplasia of the cerebellar vermis and the left cerebellar hemisphere, along with bilateral optic nerve atrophy. In the same year, the patient sustained polytraumatic injuries from a car accident and was admitted to the hospital in a comatose state for 21 days. Recurrent epileptic seizures complicated his condition. By 2024, electroencephalography (EEG) showed regular bioelectrical activity, and anticonvulsant therapy was discontinued.
Neurological examination revealed a Glasgow Coma Scale (GCS) score of E4V5M6. The patient was alert and oriented to person, place, and time. Horizontal nystagmus was observed, along with left abducens (VI cranial nerve) palsy. Other oculomotor functions were preserved. The nasolabial folds were symmetrical, and the gag reflex was intact. Muscle stretch reflexes were 5/2, and muscle tone was normal. Sensory examination revealed no abnormalities in pain, temperature, light touch, proprioception, vibration, or discriminative sensations.
Cerebellar testing revealed essential tremor during finger-to-nose testing, which was more pronounced on the left side. The heel-to-shin test was impaired, and adiadochokinesia was present. The Romberg test was negative. No meningeal signs were detected.
Baseline ophthalmologic evaluations included optical coherence tomography (OCT), which showed thinning of the retinal ganglion cell layer. Humphrey 24-2 visual field testing revealed preserved vision in one quadrant of the right eye and total visual field loss in the left eye. Visual evoked potentials (VEP) and electroretinography (ERG) showed decreased responses, consistent with severe optic nerve dysfunction. Intraocular pressure (IOP) was within normal limits both before the procedure and during the observation period.
Autologous bone marrow-derived stem cell transplantation was performed. On the day of the procedure, bone marrow aspiration was conducted under general anesthesia in a sterile operating room. A total of 120 mL of bone marrow, adjusted for the patient's age and weight, was aspirated from the anterior superior iliac spine using a bone marrow aspiration needle. The sample was collected into anticoagulated tubes for further processing.
Mononuclear cells (MNCs) were isolated using Ficoll-Paque gradient centrifugation in a cleanroom setting at Mardaleishvili Medical Center. The bone marrow was diluted 1:1 with phosphate-buffered saline (PBS), layered over Ficoll-Paque in 50 mL tubes, and then centrifuged at 2000 rpm for 10 minutes. The interphase layer was collected, washed three times with saline, and resuspended in 2.5% albumin to a final cell density of 2–10 × 10¹⁰ cells/L. Cell counts were determined using a standard hemocytometer.
Flow cytometric analysis (BD FACSCalibur) was used to characterize MNCs, mesenchymal stem cells (MSCs), and hematopoietic stem cells (HSCs). For HSC quantification, a CD34PE/CD45FITC antibody combination was used (Miltenyi Biotec). For MSC characterization, a CD45PerCP/CD105PE/CD90FITC combination was applied.
A total of 735 million hematopoietic stem cells were isolated. Of these, 490 million cells (in a 1 mL solution) were administered intrathecally. Additionally, 15 million cells were injected intravitreally into each eye (50 µL solution), 15 million cells were delivered under the episcleral region of each eye (50 µL), and 72.5 million cells were injected into the retrobulbar space bilaterally.
Within 48 hours post-transplantation, the patient reported subjective visual improvement in the right eye. Follow-up evaluations were conducted at 3, 6, and 12 months after transplantation.
Electrophysiological assessments were conducted using the Tomey system. Before transplantation, VEPs were tested using flash and pattern stimuli (1° and 0.3°). Tests were performed separately for each eye. Pattern electroretinography (PERG) ratios were used to assess retinal ganglion cell function. Retinal cone and rod activity was evaluated by ISCEV standards.
DISCUSSION
Perimetry results demonstrated notable changes over the observation period. Prior to transplantation, vision was preserved only in one quadrant. At 6 months post-transplantation, visual fields improved significantly, with vision present in all four quadrants. However, by the 12-month mark, some visual field constriction was noted compared to the 6-month result, although it remained improved relative to baseline. In summary, peripheral vision improved after stem cell transplantation, reaching its peak at 6 months, followed by partial regression by 12 months, yet still showing better results than before treatment (Fig.1).
Optical coherence tomography (OCT) results showed no significant structural changes throughout the entire observation period.
Central visual acuity in the right eye (BCVOD) fluctuated during the study period. The baseline visual acuity was 0.1, which improved to 0.4 at 6 months post-transplantation, then decreased to 0.2 by the 12-month follow-up. In contrast, left eye visual acuity (BCVOS) showed no improvement and remained at the level of hand motion from baseline to 12 months (Table 1).
Intraocular pressure (IOP) remained within normal limits throughout the study period.
FIGURE 1. The Humphrey Visual Field 24-2 SITA Standard test before and after transplantation, showing visual field changes. At 6 months, visual field results show improvement compared to the baseline, and this improvement is sustained after 12 months, with results better than the baseline

Abbreviations: OD, oculus dexter; OU, Oculus uterque.
TABLE 1. Visual evoked potential on flash stimulus
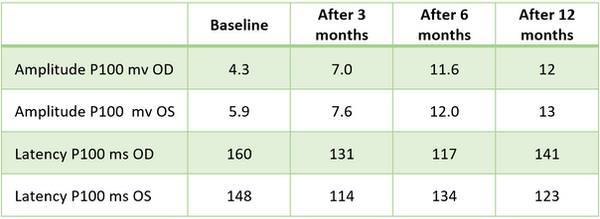
Abbreviations: OD, oculus dexter; OS, oculus sinister.
Visual evoked potentials (VEP)
The amplitude of the P100 component under flash stimulus before transplantation was OD: 4.3 μV and OS: 5.9 μV. After 1 month, the amplitude increased, continuing to rise at 6 and 12 months to OD: 11.6 μV and OS: 12.0 μV, indicating enhanced cortical response.
Latency of the P100 component was prolonged at baseline; after transplantation, the values progressively decreased and approached normal limits.
VEP responses to pattern stimuli (1° and 0.3°) also showed initial improvement. For the right eye, amplitudes increased after 1 month and peaked at 6 months (3.2 μV and 3.5 μV, respectively). In the left eye, the P100 amplitude initially increased; however, the responses at 1° and 0.3° later showed a decline. The 1° pattern response was relatively preserved in the right eye after 6 months. By 12 months, a decrease in amplitudes were noted in both eyes (Tab.2).
TABLE 2. Visual potential on the pattern stimulus ratio

Abbreviations: OD, oculus dexter; OS, oculus sinister; PERG, pattern electroretinography.
Time markers for latency exhibited mild elongation after 3 months, with this trend persisting throughout follow-up. By 12 months, latency values stabilized, suggesting adaptive cortical changes.
Ganglion cell function assessed via the PERG ratio showed an initial decrease in amplitude after transplantation, followed by an upward trend at 6 months. However, at the 12-month mark, amplitudes again declined (Tab.3), indicating potential waning of functional improvements over time.
TABLE 3. Pattern electroretinography results

Abbreviations: OD, oculus dexter; OS, oculus sinister.
Electroretinography (ERG)
Before transplantation, ERG revealed supernormal amplitudes in both rod (maximal response) and cone (30 Hz flicker) responses. Over the observation period, these amplitudes gradually declined to normal physiological levels (Tab.4), potentially reflecting a rebalancing of retinal excitability.
Subjectively, the patient reported improvement in vision within 48 hours post-transplantation. This early response is likely mediated by growth factor signaling and interaction with host neural or glial cells.
TABLE 4. Electroretinogram standard test results

Abbreviations: OD, oculus dexter; OS, oculus sinister.
Over one year following autologous bone marrow-derived stem cell transplantation, the patient experienced a 10% improvement in visual acuity, along with a marked improvement in visual fields. However, some electrophysiological and functional outcomes peaked at 6 months and regressed slightly by 12 months. This raises the question of whether observed visual improvements were primarily due to neurotrophic and paracrine effects of the transplanted cells or whether actual cell replacement and structural regeneration occurred.
CONCLUSIONS
Autologous bone marrow-derived stem cell transplantation in patients with optic nerve damage shows improvements in visual function. The changes have a transient effect. Treatment shows improvement after 1 year, as compared to the visual functions before transplantation; however, the best results are noted at 6 months after transplantation. Further investigations are necessary to elucidate the precise mechanism by which stem cells function in host tissues. Understanding the exact mechanism for the positive effect will guide the clarification of whether stem cell treatment should be a single procedure or whether continuing, intermittent transplantations are needed to sustain the positive results. Is stem cell transplantation more effective than using exosomes from the same cells that contain growth factors? The effectiveness of each transplantation area (intrathecal, retrobulbar) should be investigated.
AUTHOR AFFILIATION
1Department of Ophthalmology, Innova Medical Center, Tbilisi, Georgia;
2Mardaleishvili Medical Center, Tbilisi, Georgia;
3Department of Eye Diseases, Tbilisi State Medical University, Tbilisi, Georgia;
4Eye Clinic "Akhali Mzera", Tbilisi, Georgia;
5Department of Pathophysiology, Tbilisi State Medical University, Tbilisi, Georgia;
6Department of Biomedicine, Georgian-American University, Tbilisi, Georgia;
7Aversi Clinic, Tbilisi, Georgia;
8Department of Neurology, Tbilisi Institute of Medicine, Tbilisi, Georgia;
9Tbilisi State University, Tbilisi, Georgia;
10Faculty of Medicine, Tbilisi State Medical University, Tbilisi, Georgia;
11European School, Tbilisi, Georgia;
12Lahti General Upper Secondary School Gaudia (national and IB), Lahti, Finland
REFERENCES
-
Chaibakhsh S, Sadoughi F, Sheikhtaheri A, et al. Evaluating the impact of mesenchymal stem cell therapy on visual acuity and retinal nerve fiber layer thickness in optic neuropathy patients: a comprehensive systematic review and meta-analysis. BMC Ophthalmol. 2024;24(1):316. doi:10.1186/s12886-024-03413-2.
-
Lapshin EV, Orekhova AS, Korostyleva EV, Terskikh VV. The potential and application of iPSCs in gene and cell therapy for retinopathies and optic neuropathies. Acta Naturae. 2023;15(4):56-68.
-
Chang KYC, Chiang PH, Lin TTY, Chen KH. Induced pluripotent stem cells for inherited optic neuropathies. J Neuroophthalmol. 2022;42(1):30-36. doi:10.1097/WNO.0000000000001230.
-
Lee J, Nguyen S, Bhattacharya S. Optic nerve regeneration: potential treatment approaches. Curr Opin Pharmacol. 2024;74:102428. doi:10.1016/j.coph.2023.102428.
-
De Gioia R, Biella F, Citterio G, et al. Neural stem cell transplantation for neurodegenerative diseases. Int J Mol Sci. 2020;21(9):3103. doi:10.3390/ijms21093103.
-
Ge H, Zhang D, Wei H, et al. Cell therapy for optic nerve injury: mesenchymal stem cells and beyond. Stem Cell Rev Rep. 2021;17(5):1510-1528. doi:10.1007/s12015-021-10164-9.
-
Eslami Z, Ghiasi M, Hassani SN, et al. Stem cell-based therapies for optic neuropathies: current approaches and future directions. Cell Tissue Res. 2021;383(3):945-959. doi:10.1007/s00441-020-03295-0.
-
Beike Biotechnology. Stem cell treatment for optic nerve atrophy. Beike Biotechnology website. https://beikecelltherapy.com/treatments/stem-cell-treatment-optic-nerve-atrophy. Published 2023. Accessed June 17, 2025.
-
Yamashita T, Ninomiya M, Katayama T, et al. Neural regeneration and plasticity after optic nerve injury in animals. Neural Regen Res. 2020;15(9):1640-1646. doi:10.4103/1673-5374.275338.
-
Kuwahara S, Ohta S, Nagai N. iPSC-based retinal therapy: challenges and future perspectives. Regen Med. 2022;17(4):223-234. doi:10.2217/rme-2021-0156.
-
Shokouhi M, Norooznezhad AH, Asadnia M, et al. Exosome-based delivery for retinal and optic nerve regeneration. Biomedicines. 2024;12(2):256. doi:10.3390/biomedicines12020256.
-
Kolar MK, Itte VN, Kingham PJ, Reid AJ. The neurotrophic effects of different human dental stem cells. Sci Rep. 2017;7:12605. doi:10.1038/s41598-017-12836-z.
-
Bhat S, Viswanathan P, Chandanala S, et al. Expansion and characterization of bone marrow-derived human stromal cells in serum-free conditions. Sci Rep. 2021;11:3403. doi:10.1038/s41598-021-82880-3
