ORIGINAL RESEARCH
Assessment of Gross Motor Function in Children with Spastic Cerebral Palsy Before Treatment and Two Years After Medical Rehabilitation
Mariam Sopromadze1, Irakli Natroshvili2,ID
ABSTRACT
Background: Spastic cerebral palsy (spastic tetraplegia) is an irreversible brain injury that causes motor dysfunction, sensory disturbances, and cognitive impairments in children. Motor dysfunction leads to difficulties with balance when standing, sitting, and walking. Movements are often chaotic and uncoordinated. Although it results from a brain injury and is unpredictable, motor problems may worsen as the child grows, making early physical rehabilitation and occupational therapy essential. Patients with spastic tetraplegia frequently face vision and hearing impairments, intellectual and behavioral issues, lack tactile sensations, and experience speech deficits.
Objectives: The study focused on children with spastic cerebral palsy. It involved patients who were relatively compliant with treatment and not severely affected by the condition. The study included 33 patients, divided into four age groups: nine patients aged 5-6 years, eight patients aged 7-8 years, nine patients aged 9-10 years, and seven patients aged 11-12 years.
Methods: Patients underwent a Gross Motor Function Measure (GMFM). The outcomes were quantitative values (in percentages) for six qualitative characteristics: (i) Lying and rolling; (ii) Sitting; (iii) Crawling and kneeling; (iv) Standing; (v) Walking; and (vi) Total gross motor function score. The GMFM-88 scale was used, with each item scored on a scale of 0 to 3. The severity of spastic diplegia was assessed based on independent mobility, categorizing disease severity into five levels.
Results: Statistical analysis showed a significant difference (p < 0.05) in gross motor function scores between the pre-treatment period and two years after rehabilitation. When comparing age groups 5-6 and 7-8 years, all six characteristics displayed significant differences after two years of treatment (U < U_{crit}, p < 0.05), indicating better outcomes in the 5-6-year-old group. Before treatment, the comparison between age groups 7-8 and 9-10 found no significant differences in the second (sitting) and fourth (standing) characteristics (U > U_{crit}, U_{crit} = 15, p > 0.05). The other four characteristics showed significant differences (U < U_{crit}, p < 0.05). After two years, all six characteristics significantly improved in favor of the 7-8-year-old group (U < U_{crit}, p < 0.05). Comparing the third (crawling and kneeling) and fifth (walking) characteristics (U > U_{crit}, p > 0.05). However, the remaining four characteristics showed significant differences (p < 0.05). After two years of treatment, all six characteristics were significantly better in the 9- to 10-year-old group (U < U_{crit}, p < 0.05).
Conclusions: In patients diagnosed with spastic cerebral palsy, especially spastic tetraplegia, treatment planning should go beyond just age and sex. Instead, a personalized approach must be based on a thorough multidisciplinary assessment. This evaluation, done collaboratively by various healthcare professionals, should focus on identifying and prioritizing the patient's most affected functional areas, ensuring interventions target the most critical needs. In addition to patient-specific clinical factors, it is also essential to consider the unique sociocultural and healthcare conditions of the country where the patient resides. Our study clearly shows a trend: the earlier a structured two-year rehabilitation program begins, containing 384 therapeutic procedures, the greater the improvement in gross motor function scores. This highlights the importance of early intervention in managing spastic cerebral palsy. Regular monitoring of gross motor function outcomes throughout treatment provides a complete picture of the patient's progress. Such data help clinicians determine which areas are improving significantly and which may require increased attention or adjustments to therapy.
Keywords: Age-related outcomes; GMFM-88; gross motor function; pediatric rehabilitation; physical therapy; spastic tetraplegia.
DOI: 10.52340/GBMN.2025.01.01.118
BACKGROUND
Spastic quadriplegic cerebral palsy (CP) is a permanent neuromuscular disorder characterized by motor impairment affecting all four limbs, resulting from a lesion in the developing brain. Most children with spastic quadriplegic CP are classified as level V on the Gross Motor Function Classification System (GMFCS), indicating the most severe degree of functional limitation. This classification is associated with a higher rate of comorbidities compared to other CP types at lower GMFCS levels.1
Weak and inactive postural muscles in the neck and trunk characterize spastic quadriplegic CP. As a compensatory mechanism, children often adopt a posture of full body extension, leading to unusual movement patterns. These maladaptive patterns cause multisystem effects that gradually reduce the quality of life for affected individuals.1
Disturbances in sensation, perception, and cognition frequently accompany the motor impairments associated with cerebral palsy. Additionally, many children face communication challenges, behavioral disorders, epilepsy, and secondary musculoskeletal complications.2
The prevalence of cerebral palsy worldwide ranges from about 1.5 to 3 per 1,000 live births, with significant variation across different regions and economic levels. Rates tend to be higher in low- and middle-income countries (LMICs) compared to high-income countries.3 In LMICs, birth asphyxia leading to hypoxic-ischemic encephalopathy (HIE) remains the primary cause of CP, closely followed by neonatal jaundice. Other known risk factors include prematurity, congenital brain malformations, and genetic or metabolic disorders.4 Birth asphyxia and HIE can cause multiorgan dysfunction, including issues with the heart, kidneys, liver, and blood, which may persist into later childhood stages.4
Cerebral palsy is typically classified based on the type of movement disorder (spastic, athetoid, ataxic, or mixed), the distribution of affected limbs (hemiplegia, diplegia, or quadriplegia), and the level of functional impairment (mild, moderate, severe, or profound). Spastic cerebral palsy, the most common subtype, includes several clinical variants. In spastic hemiplegia or hemiparesis, one side of the body—usually the arm, hand, and sometimes the leg—is affected. Children with this type generally have preserved cognitive function, although speech delays can occur. Spastic diplegia or diparesis primarily affects the lower limbs, with less involvement of the arms and face; cognitive and language development are typically normal. Spastic quadriplegia or quadriparesis, the most severe form, features significant weakness in the neck and severe rigidity of all four limbs. These children are usually unable to walk and often have severe speech impairments.5
In children with spastic quadriplegic CP, the inability to bring the head to midline and hold it there, or to perform chin-tucking (capital flexion) while supine, results from weak and inactive postural muscles in the neck and trunk. To stabilize the head, these children may use compensatory mechanisms such as scapular elevation, adduction, and internal rotation, which further restrict normal head and neck movement. Additionally, they often lack control over scapular positioning on the thorax and cannot perform active extension through the thoracic spine. These deficits prevent effective weight-bearing with the forearm and extended arm, thereby reducing proprioceptive input, which is critical for motor development. Without the ability to shift their weight normally, these children often adopt maladaptive patterns involving cervical extension, scapular adduction, and total body extension, which hinder further motor progress.1
METHODS
The research involved patients with spastic cerebral palsy. The study included patients who were relatively responsive to treatment and not severely affected. The study involved 33 patients, comprising nine aged 5-6 years, eight aged 7-8 years, nine aged 9-10 years, and seven aged 11-12 years. The null hypothesis (H₀) was used to test whether there were significant differences between the groups.
Patients underwent an assessment of gross motor function (GMFM). The results were presented as percentage scores for six qualitative categories: (i) Lying and rolling; (ii) Sitting; (iii) Crawling and kneeling; (iv) Standing; (v) Walking; and (vi) the total gross motor function score.
Patients underwent comprehensive rehabilitation therapy tailored with individualized treatment plans created through an interdisciplinary approach, considering disease severity and treatment goals.
The treatment process involved a multidisciplinary team comprising a rehabilitation physician, neurologist, physical therapist, occupational therapist, speech therapist, orthopedic specialist, and orthotist. These professionals completed a four-year training program in Georgia as part of a national initiative to enhance physical rehabilitation services, conducted under the guidance of experts from Emory University in Atlanta, USA. The treatment administered to the patients in the study was conducted under the direct supervision of these specialists and spanned two years. During this period, each patient underwent 40 treatment cycles, amounting to 384 rehabilitation procedures.
The study employed the Gross Motor Function Measure (GMFM) as its primary assessment tool. Specifically, the GMFM-88 version was used, which assesses six qualitative areas of gross motor function. Each item was scored from 0 to 3 points, depending on the extent to which the task was completed. When evaluating the severity of spastic diplegia, special focus was given to the child's ability to move independently. Based on these functional measures, the severity of the condition was categorized into five distinct levels.
Statistical analysis was conducted using SPSS. To evaluate the null hypothesis (H₀), the Mann-Whitney U test was employed due to the unknown distribution, with a significance level of 0.05 (95% confidence) and degrees of freedom v = 14. The critical U value was set at 13.
RESULTS
The difference between pre-treatment and post-treatment after two years was statistically significant (p<0.05). In all age groups, mean values after treatment significantly exceeded the baseline means. Additionally, the study compared pre-treatment (sample I) and post-treatment (sample II) results by age group and qualitative features, presenting the results as percentages.
Table 1 shows the Gross Motor Function Measurement (GMFM) results before and after two years of treatment in 5- to 6-year-old patients with spastic cerebral palsy (spastic tetraplegia).
TABLE 1. Gross Motor Function Measurement (GMFM) results before and after treatment in 5- to 6-year-old patients

The results of the Gross Motor Function Measurement (GMFM) before and after two years of treatment in 7- to 8-year-old patients with spastic cerebral palsy (spastic tetraplegia) are shown in Table 2.
TABLE 2. Gross Motor Function Measurement (GMFM) results before and after treatment in 7- to 8-year-old patients

The results of the pre- and post-treatment GMFM in 9- to 10-year-old patients with spastic cerebral palsy (spastic tetraplegia) are presented in Table 3.
TABLE 3. Gross Motor Function Measurement (GMFM) results before and after treatment in 9- to 10-year-old patients

Table 4 shows the results of GMFM before and after two years of treatment in 11- to 12-year-old patients with spastic cerebral palsy (spastic tetraplegia).
TABLE 4. Gross Motor Function Measurement (GMFM) results before and after treatment in 9- to 10-year-old patients
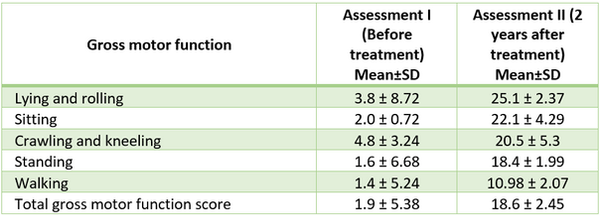
Comparing the 5-6 and 7-8 age groups before treatment, no significant differences were observed in the third (crawling and kneeling), fourth (standing), and fifth (walking) features. Since U critical = 15, and U > Ucrit in these cases (P > 0.05), the results were not significant. However, two years after treatment, significant differences were found across all six qualitative parameters (U < Ucrit, P < 0.05), with better outcomes in the 5- to 6-year-old age group.
Before treatment, a statistical comparison between the 7-8 and 9-10-year-old age groups showed that differences in the second (sitting) and fourth (standing) qualitative parameters were not statistically significant. For these parameters, the condition U > U₍cᵣᵢₜ₎ (U₍cᵣᵢₜ₎ = 15, P > 0.05) was met. However, for the remaining four qualitative parameters, the differences were statistically significant, as the results met the condition U < U₍cᵣᵢₜ₎ (P < 0.05). Two years after starting treatment, comparison of both age groups revealed statistically significant differences across all six qualitative parameters, favoring the 7-8-year-old group. For each parameter, the condition U < U₍cᵣᵢₜ₎ (P < 0.05) was satisfied, indicating that patients in the 7-8-year-old group responded more effectively to treatment than those in the 9-10-year-old group.
Before treatment, a statistical comparison between the 9-10 and 11-12-year-old age groups showed no statistically significant differences in the third (crawling and kneeling) and fifth (walking) qualitative parameters. In these cases, the condition U > U₍cᵣᵢₜ₎ (P > 0.05) was satisfied. However, for the remaining four qualitative parameters, the differences were statistically significant.
Two years after starting treatment, a comparison between the two age groups showed statistically significant differences across all six qualitative parameters, favoring the 9-10-year-old group. In each case, the condition U < U₍cᵣᵢₜ₎ (P < 0.05) was met, indicating that post-treatment outcomes were more favorable in patients aged 9-10 years compared to those aged 11-12 years
DISCUSSION
Physiotherapy plays a vital role in managing cerebral palsy (CP), and nearly all individuals diagnosed with CP undergo physiotherapy interventions. The primary goals of physiotherapy are to support the child's participation in daily activities and to address the physical impairments associated with the condition. Through targeted treatments, physiotherapy helps children with CP achieve their highest level of physical independence, maintain optimal fitness, and improve overall quality of life - not only for the child but also for their families—by reducing the functional impact of motor impairments.6
Managing CP requires a comprehensive, multidisciplinary approach. After establishing the diagnosis, the infant or child should undergo a thorough evaluation by a coordinated rehabilitation team. The team’s composition may vary depending on the clinical setting and the availability of resources. Core team members might include a physiatrist, developmental pediatrician, orthopedic surgeon, neurologist, physical therapist, occupational therapist, speech-language pathologist, therapeutic recreation specialist, orthotist, psychologist, social worker, and nutritionist.
Collaboration between the team and the child's caregivers is crucial for developing both short-term and long-term goals. These goals should address neuromuscular issues such as maintaining range of motion (ROM) and managing muscle tone, as well as functional areas like self-care, mobility, and communication. Goals aimed at enhancing the child's participation in community and societal activities should also be prioritized. Regular reassessment is essential to keep these goals relevant as the child grows. When appropriate, the child should be actively involved in the goal-setting process to promote autonomy and engagement.
Once individualized goals are established, the rehabilitation team and the family must select the most suitable therapeutic strategies to achieve these goals. Although various treatment options are available, the scientific evidence supporting specific interventions remains limited. This is mainly due to the variability of CP presentations, the lack of strong control groups in studies, and the absence of condition-specific outcome measures. In clinical practice, treatment typically begins with the least invasive and most cost-effective interventions, with continuous evaluation to guide and adjust therapeutic choices.7
In children with spastic cerebral palsy (spastic tetraplegia), those aged 5-6 and 7-8 often exhibit improved functional abilities and compensatory skills, resulting in higher GMFM scores.
These patients often experience pain caused by severe musculoskeletal deformities, muscle rigidity, weakness, scoliosis, and respiratory problems. After treatment, they may gain partial independence in sitting and walking. If not, wheelchairs and adaptive devices are essential. Speech issues might require augmentative and alternative communication (AAC) methods, such as gesture language and speech therapy. Dysphagia is also common.
The rehabilitation program must be developed considering both the child's and the parent's interests. Patient assessment should also occur in the home environment. The occupational therapist should evaluate which toys the child prefers and use this information to guide therapy. The child should gradually become familiar with their surroundings; it is important not to overwhelm or pressure them during the adaptation process.
Building rapport with the child is essential. Therapists should speak in a calm tone and smile during interactions. Items should be visible to the child, placed nearby, and adjusted according to the child's posture and movements. According to Levitt's approach, children should be gradually introduced to social activities whenever possible, such as waving goodbye and using simple social cues. The child should play with colorful toys, but these toys should not be labeled or named during therapy sessions.
Patients often do not engage in daily activities. If the child cannot stand on their own, a table or chair can be used as support.
Occupational therapists should help the child touch different body parts, especially the face and mouth. Sensory awareness can be enhanced through feeding. However, spastic tetraplegia is the most severe form of cerebral palsy, and some children may never achieve independent oral feeding.
CONCLUSIONS
Patients with spastic cerebral palsy, specifically spastic tetraplegia, who are under clinical observation, should receive treatment based not only on age and sex but also through an individualized approach developed from comprehensive evaluations by a multidisciplinary team. The treatment plan should focus on the most significant functional impairments identified during these evaluations. Additionally, the specific sociocultural and healthcare conditions of the country should be taken into account when designing rehabilitation strategies.
Our study found that the earlier a structured two-year treatment program (comprising 384 procedures) is initiated, the greater the improvement in gross motor function outcomes, as indicated by percentage increases in functional scores.
Monitoring the results of gross motor function assessments gives clinicians a clear understanding of the patient's progress. This enables them to pinpoint which areas of motor function are improving and which require additional therapeutic attention.
AUTHOR AFFILIATION
1 Department of Odontology, Tbilisi State Medical University, Tbilisi, Georgia
2 Ken Walker University Clinic for Medical Rehabilitation, Tbilisi, Georgia
ACKNOWLEDGEMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
REFERENCES
-
Wahyuni, L. K. (2023). Multisystem compensations and consequences in spastic quadriplegic cerebral palsy children. Frontiers in Neurology, 13, 1076316. https://doi.org/10.3389/fneur.2022.1076316.
-
Tedla, J. S., & Reddy, R. S. (2021). Evaluation of psychometric properties of the segmental assessments of trunk control (SATCO) in children with spastic quadriplegia cerebral palsy. Nigerian Journal of Clinical Practice, 24(7), 1077–1081.
-
Patel, D. R. (2024). Cerebral palsy in children: A clinical practice review. Current Problems in Pediatric and Adolescent Health Care, 54(11), 101673.
-
Kija, E. (2025). Management of cerebral palsy in low- and middle-income countries. Seminars in Pediatric Neurology. Advance online publication. https://doi.org/10.1016/j.spen.2025.101202.
-
Basoya, S. (2023). Cerebral palsy: A narrative review on childhood disorders. Cureus.
-
Das, S. P. (2019). Evidence-based approach to physical therapy in cerebral palsy. Indian Journal of Orthopaedics, 53(1), 20–34. https://doi.org/10.4103/ortho.IJOrtho_241_17.
-
Alexander, M. A. (2015). Cerebral palsy. In M. A. Alexander & D. A. Matthews (Eds.), Pediatric rehabilitation (5th ed., pp. 348–349). Demos Medical Publishing.
